The neuronal protein Tau and its complex phosphorylation code
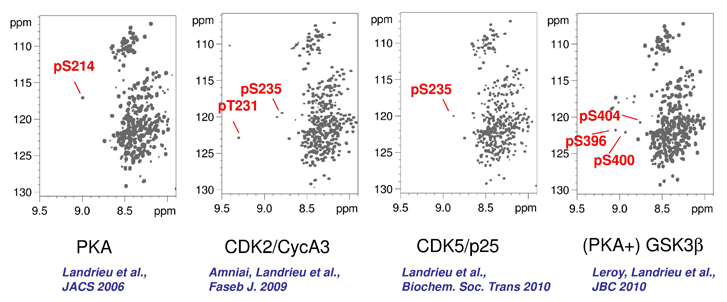
We are focusing on the deciphering of the complex phosphorylation code of Tau and its implication in Alzheimer Disease. We combine in vitro enzymatic reactions with recombinant kinases to phosphorylate the neuronal Tau protein, and Nuclear Magnetic Resonance spectroscopy to unravel the resulting phosphorylation pattern. Beyond the analytical capacity, this approach is followed by functional assays with the same samples to characterize protein-protein interactions (PPI) depending on specific phosphorylation patterns of Tau. We have shown for example that Tau interaction with 14-3-3 specifically depends on PKA phosphorylation. Some of these phospho-specific PPI will in turn regulate further post-transcriptional modifications (PTM) of Tau, such as the interaction with the phospho-dependent prolyl cis/trans isomerase Pin1 that modulates Tau dephosphorylation by the PP2A phosphatase. In addition to deciphering the complex biology of Tau, this methodology can also be used to characterize the phospho-epitopes recognized by monoclonal antibodies.
Contact: isabelle.landrieu@univ-lille.fr