Current Members

Davy Sinnaeve
CNRS Researcher / PI Contact

Wiktor Adamski
Postdoctoral Researcher Contact

To be recruited
Ph.D student Contact
Research
Pulse sequence development
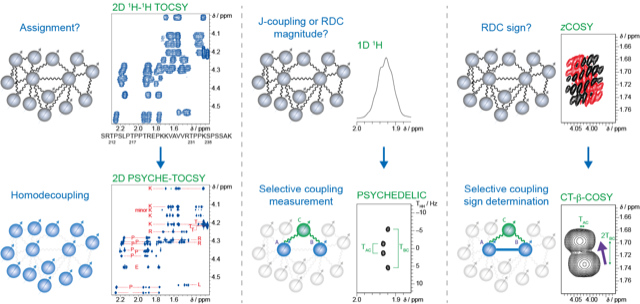
NMR spectroscopy is ideally suited to investigate Intrinsically disordered proteins (IDPs) or disordered protein regions (IDRs). As a technique, it is very sensitive to changes in molecular dynamics, and the inherent disorder incurs favourable spectroscopic properties that allow the design of very informative experiments. However, IDPs also present unique challenges to NMR spectroscopy. Compared to their globular counterparts, IDPs display significantly lower chemical shift dispersion, leading – even for small proteins – to very crowded NMR spectra, impairing extraction of valuable structural information. Moreover, the fast interconversion between conformers makes data interpretation not straightforward. We are interested in developing new approaches that harvest a maximum of spectral information that can be translated into structural information, such as residual dipolar couplings (RDCs). Central to this is the use of ‘pure shift’ techniques, whereby the signals are homodecoupled so that multiplets in the 1H spectrum of typically 10-50 Hz wide reduce to singlets of only a few Hz wide, improving resolution by an order of magnitude. Such methods have proven very valuable for small molecules, including the measurement of 1H-1H scalar couplings and RDCs (Sinnaeve et al. Angew. Chem. Int. Ed. 2015; Anal. Chem. 2018; Angew. Chem. Int. Ed. 2020), and also hold potential for IDPs.
Recent papers:
Sinnaeve D., Selective Homonuclear 2D J-resolved Spectroscopy, Emagres (2021) 9(4):267-281
Sinnaeve D., Ilgen J., Di Pietro M.E., Primozic J.J., Schmidts V., Thiele C.M. and Luy B., Probing Long-Range Anisotropic Interactions: a General and Sign-Sensitive Strategy to Measure 1H-1H Residual Dipolar Couplings as a Key Advance for Organic Structure Determination, Angewandte Chemie-International Edition (2020) 59(13):5316-5320
Sinnaeve D., Dinclaux M., Cahoreau E., Millard P., Portais J.C., Letisse F. and Lippens G., Improved Isotopic Profiling by Pure Shift Heteronuclear 2D J-Resolved NMR Spectroscopy, Analytical Chemistry (2018) 90(6):4025-4031
Sinnaeve D., Clean pure shift 2D J-resolved spectroscopy, Magnetic Resonance in Chemistry (2018) 56(10):947-953
Proline-rich sequences and fluorinated amino acids
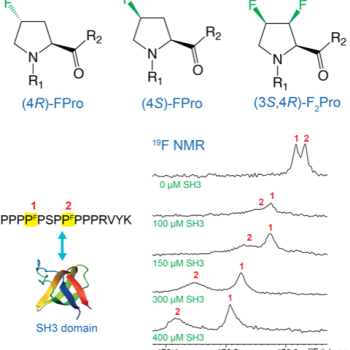
We are particularly interested in investigating the structure-functional relationships of proline-rich motifs (PRMs) and oligoproline stretches, which are found abundantly in IDPs. PRMs are key to protein-protein interactions (involving for instance SH3 domains), and mediate biological processes such as cell signaling or neurodegenerative diseases. Examples of PRMs are found in the proteins Huntingtin (implicated in Huntington disease) and tau (implicated in Alzheimer disease). The unique features of proline — the lack of an amide proton, its aliphatic five-membered ring, and the cis/trans peptide bond isomerization — make PRMs particularly challenging for NMR study. Besides the use of homodecoupling techniques, we are interested in the use of fluorinated prolines to investigate the role of individual proline conformations and exploit 19F NMR for interaction studies.
Recent papers:
Sinnaeve D., Ben Bouzayene A., Ottoy E., Hofman G.J., Erdmann E., Linclau B., Kuprov I., Martins J.C., Torbeev V. and Kieffer B., Fluorine NMR study of proline-rich sequences using fluoroprolines, Magnetic Resonance (2021) 2(2):795-813
Linclau B., Wang Z., Jeffries B., Graton J., Carbajo R.J., Sinnaeve D., Le Questel J.Y., Scott J.S., Chiarparin E., Relating conformational equilibria to conformer-specific lipophilicities: new opportunities in drug discovery, Angewandte Chemie-International Edition (2022) 61(7):e202114862
Hofman G.J., Ottoy E., Light M.E., Kieffer B., Martins J.C., Kuprov I., Sinnaeve D. and Linclau B., Synthesis and Conformational Properties of 3,4-Difluoro-L-prolines, Journal of Organic Chemistry (2019) 84(6):3100-3120
Hofman G.J., Ottoy E., Light M.E., Kieffer B., Kuprov I., Martins J.C., Sinnaeve D. and Linclau B., Minimising conformational bias in fluoroprolines through vicinal difluorination, Chemical Communications (2018) 54(40):5118-5121
